Discovery of novel trimethoxy-ring BRD4 bromodomain inhibitors: AlphaScreen assay, crystallography and cell-based assay†‡
Abstract
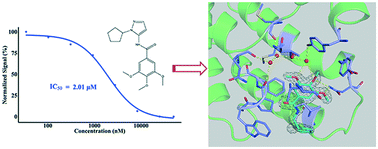
As a member of the bromodomain and extra terminal domain (BET) protein family, BRD4 is closely related to cancers and other diseases. Small-molecule BRD4 inhibitors have already demonstrated promising potential for the therapy of BRD4-related cancers. In this study, we report the discovery and evaluation of a novel category of BRD4 inhibitors, which share a trimethoxy ring and target the first bromodomain of the human BRD4 protein. The IC50 value of the most potent compound, DC-BD-03, is 2.01 μM. In addition, a high-resolution crystal structure of the compound DC-BD-29 with the first bromodomain of BRD4 was determined, which revealed the binding mode and facilitated further structure-based optimization. These compounds exhibited anti-proliferation activity, caused cell cycle arrest, and induced apoptosis in human leukemia MV4-11 cells. Thus, the results presented in this study indicated the potential of this series of compounds as drug candidates for the therapy of BRD4-related cancers.


 Please wait while we load your content...
Please wait while we load your content...