Abstract
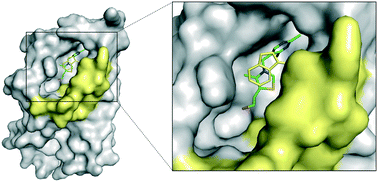
Energy-coupling factor (ECF) transporters are involved in the uptake of micronutrients in bacteria. The transporters capture the substrate by high-affinity binding proteins, the so-called S-components. Here, we present the analysis of two regions of the substrate-binding pocket of the thiamine-specific S-component in Lactococcus lactis, ThiT. First, interaction of the thiazolium ring of thiamine with residues Trp34, His125 and Glu84 by π–π-stacking and cation–π is studied, and second, the part of the binding pocket that extends from the hydroxyl group. We mutated either the transported ligand (chemically) or the protein (genetically). Surprisingly, modifications in the thiazolium ring by introducing substituents with opposite electronic effects had similar effects on the binding affinity. We hypothesize that the electronic effects are superseeded by steric effects of the added substituents, which renders the study of isolated interactions difficult. Amino acid substitutions in ThiT indicate that the electrostatic interaction facilitated by residue Glu84 of ThiT and thiamine is necessary for picomolar affinity. Deazathiamine derivatives that explore the subpocket of the binding site extending from the hydroxyl group of thiamine bind with high affinity to ThiT and may be developed into selective inhibitors of thiamine transport by ECF transporters. Molecular-dynamics simulations suggest that two of these derivatives may not only bind to ThiT, but could also be transported.


 Please wait while we load your content...
Please wait while we load your content...