Histone lysine methyltransferase structure activity relationships that allow for segregation of G9a inhibition and anti-Plasmodium activity†‡
Abstract
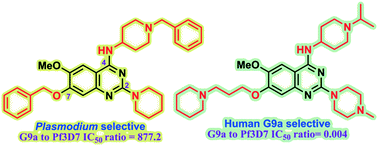
Plasmodium falciparum HKMTs (PfHKMTs) play a key role in controlling Plasmodium gene expression and represent exciting new anti-malarial epigenetic targets. Using an inhibitor series derived from the diaminoquinazoline HKMT inhibitory chemotype, we have previously identified compounds with highly promising antimalarial activity, including irreversible asexual cycle blood stage-independent cytotoxic activity at nM concentrations, oral efficacy in in vivo models of disease, and the unprecedented ability to reactivate dormant liver stage parasites (hypnozoites). However, future development of this series will need to address host versus parasite selectivity, where inhibitory activity against human G9a is removed from the lead compounds, while maintaining potent anti-Plasmodium activity. Herein, we report an extensive study of the SAR of this series against both G9a and P. falciparum. We have identified key SAR features which demonstrate that high parasite vs. G9a selectivity can be achieved by selecting appropriate substituents at position 2, 4 and 7 of the quinazoline ring. We have also, in turn, discovered that potent G9a inhibitors can be identified by employing a 6-carbon ‘Nle mimic’ at position 7. Together, this data suggests that while broadly similar, the G9a and potential PfHKMT target(s) binding pockets and/or binding modes of the diaminoquinazoline analogues exhibit clear and exploitable differences. Based on this, we believe this scaffold to have clear potential for development into a novel anti-malarial therapeutic.


 Please wait while we load your content...
Please wait while we load your content...