New tricks for human farnesyltransferase inhibitor: cancer and beyond†
Abstract
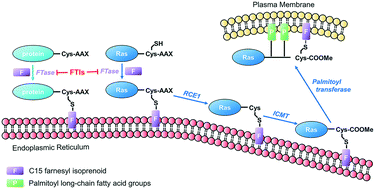
Human protein farnesyltransferase (FTase) catalyzes the addition of a C15-farnesyl lipid group to the cysteine residue located in the COOH-terminal tetrapeptide motif of a variety of important substrate proteins, including well-known Ras protein superfamily. The farnesylation of Ras protein is required both for its normal physiological function, and for the transforming capacity of its oncogenic mutants. Over the last several decades, FTase inhibitors (FTIs) were developed to disrupt the farnesylation of oncogenic Ras as anti-cancer agents, and some of them have entered cancer clinical investigation. On the other hand, some substrates of FTase were demonstrated to be related with other human diseases, including Hutchinson–Gilford progeria syndrome, chronic hepatitis D, and cardiovascular diseases. In this review, we summarize the roles of FTase in malignant transformation, proliferation, apoptosis, angiogenesis, and metastasis of tumor cells, and the recently anticancer clinical research advances of FTIs. The therapeutic prospect of FTIs on several other human diseases is also discussed.


 Please wait while we load your content...
Please wait while we load your content...