Design, synthesis and evaluation of new ligustrazine derivatives as potential plasma-stable neuroprotective agents†‡
Abstract
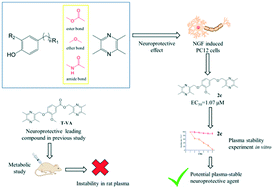
A series of ligustrazine–phenolic acid esters which exhibited promising neuroprotective activities have previously been reported. Nevertheless, we found that these ester compounds (like T-VA) were not stable in plasma by further in vivo studies. To investigate plasma-stable neuroprotective agents, a series of new ligustrazine derivatives were synthesized by conjoining ligustrazine and phenols with ester, ether and amide bonds. Most of the compounds exhibited higher protective effects against CoCl2-induced neurotoxicity in differentiated PC12 cells than ligustrazine. Structure–activity relationships were also briefly discussed. We found that compound 2c (2-((2-methoxy-4-(((3,5,6-trimethylpyrazin-2-yl)methoxy) methyl)phenoxy)methyl)-3,5,6-trimethylpyrazine) displayed the highest protective effect on the PC12 cells damaged by CoCl2 (EC50 = 1.07 μM). Preliminary stability investigation in rat plasma was verified in vitro and better plasma stability was observed with 2c in comparison to T-VA.


 Please wait while we load your content...
Please wait while we load your content...