Abstract
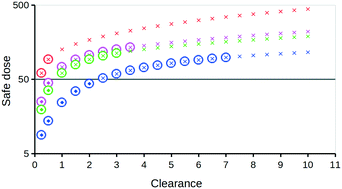
Designing an oral drug such that its estimated dose to humans is both efficacious and safe is challenging. During the early design stage, where in vitro or preclinical species in vivo safety data are limited, heuristic-based criteria often related to physicochemical properties are used for guidance. The causal relationship between a compound's log P and its human in vivo toxicity is considered. With respect to designing efficacious oral drugs that potentially have reduced toxicity liabilities, an alternative heuristic-based criterion is proposed based on the amount of compound in the body at steady state. In humans, a threshold for the amount of compound in the body at steady state of 0.5 mg kg−1 is suggested. The criterion is based on the minimum toxic blood–plasma concentration that produces clinically relevant side effects or symptoms in humans for 242 oral drugs. It can be used to estimate a therapeutic window against which a compound's estimated in vivo plasma levels for a particular dose size and frequency can be assessed. The relationship between this criterion and acceptable oral dose sizes for different charge types with different in vivo plasma clearances is discussed.


 Please wait while we load your content...
Please wait while we load your content...