Design, synthesis and biological evaluation of novel Schiff base-bridged tetrahydroprotoberberine triazoles as a new type of potential antimicrobial agents†‡
Abstract
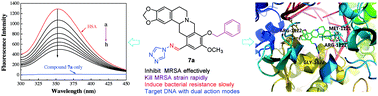
A series of novel Schiff base-bridged tetrahydroprotoberberine (THPB) triazoles were designed, synthesized and characterized for the first time. Antimicrobial assay showed that some of the prepared compounds exerted stronger antibacterial and antifungal activities than the reference drugs. Especially, THPB triazole 7a gave low MIC values of 0.5, 1 and 2 μg mL−1 against B. yeast, M. luteus and MRSA, respectively. Further experiments indicated that the highly active molecule 7a was able to rapidly kill the MRSA strain and did not trigger the development of bacterial resistance even after 14 passages. The preliminary exploration for the antimicrobial mechanism revealed that compound 7a could effectively intercalate into calf thymus DNA to form a 7a–DNA supramolecular complex, and its Zn2+ complex had the ability to directly cleave pUC19 DNA, which suggested that compound 7a might be a potentially dual-targeting antibacterial molecule. It was also found that compound 7a could be efficiently stored and carried by human serum albumin (HSA), and the hydrophobic interactions and hydrogen bonds played important roles in the transportation of HSA to the active molecule 7a.


 Please wait while we load your content...
Please wait while we load your content...