The molecular basis of the interactions between synthetic retinoic acid analogues and the retinoic acid receptors†‡
Abstract
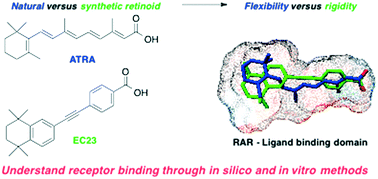
All-trans-retinoic acid (ATRA) and its synthetic analogues EC23 and EC19 direct cellular differentiation by interacting as ligands for the retinoic acid receptor (RARα, β and γ) family of nuclear receptor proteins. To date, a number of crystal structures of natural and synthetic ligands complexed to their target proteins have been solved, providing molecular level snap-shots of ligand binding. However, a deeper understanding of receptor and ligand flexibility and conformational freedom is required to develop stable and effective ATRA analogues for clinical use. Therefore, we have used molecular modelling techniques to define RAR interactions with ATRA and two synthetic analogues, EC19 and EC23, and compared their predicted biochemical activities to experimental measurements of relative ligand affinity and recruitment of coactivator proteins. A comprehensive molecular docking approach that explored the conformational space of the ligands indicated that ATRA is able to bind the three RAR proteins in a number of conformations with one extended structure being favoured. In contrast the biologically-distinct isomer, 9-cis-retinoic acid (9CRA), showed significantly less conformational flexibility in the RAR binding pockets. These findings were used to inform docking studies of the synthetic retinoids EC23 and EC19, and their respective methyl esters. EC23 was found to be an excellent mimic for ATRA, and occupied similar binding modes to ATRA in all three target RAR proteins. In comparison, EC19 exhibited an alternative binding mode which reduces the strength of key polar interactions in RARα/γ but is well-suited to the larger RARβ binding pocket. In contrast, docking of the corresponding esters revealed the loss of key polar interactions which may explain the much reduced biological activity. Our computational results were complemented using an in vitro binding assay based on FRET measurements, which showed that EC23 was a strongly binding, pan-agonist of the RARs, while EC19 exhibited specificity for RARβ, as predicted by the docking studies. These findings can account for the distinct behaviour of EC23 and EC19 in cellular differentiation assays, and additionally, the methods described herein can be further applied to the understanding of the molecular basis for the selectivity of different retinoids to RARα, β and γ.


 Please wait while we load your content...
Please wait while we load your content...