Abstract
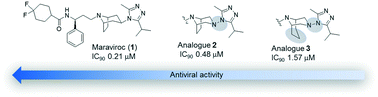
Two diazabicyclo analogues of maraviroc, in which the azabicyclooctane moiety is replaced by diazabicyclooctane or diazabicyclononane, were synthesized and tested, through a viral neutralization assay, on a panel of six pseudoviruses. The diazabicyclooctane derivative maintained a significant infectivity reduction power, whereas the diazabicyclononane was less effective. Biological data were rationalized through a computational study that allowed the conformational preferences of the compounds to be determined and a correlation between the inhibitory activity, the bridge length of the bicycle, and the rotational barrier around dihedral angle τ7 to be hypothesized. A high-field NMR analysis supported the modeling results.


 Please wait while we load your content...
Please wait while we load your content...