Abstract
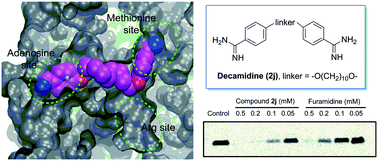
Protein arginine methyltransferase 1 (PRMT1) is a key player for the dynamic regulation of arginine methylation. Its dysregulation and aberrant expression are implicated in various pathological conditions, and a plethora of evidence suggests that PRMT1 inhibition is of significant therapeutic value. Herein, we reported the modification of a series of diamidine compounds with varied lengths in the middle alkyl linker for PRMT1 inhibition. Decamidine (2j), which possesses the longest linker in the series, displayed 2- and 4-fold increase in PRMT1 inhibition (IC50 = 13 μM), compared with furamidine and stilbamidine. The inhibitory activity toward PRMT1 was validated by secondary orthogonal assays. Docking studies showed that the increased activity is due to the extra interaction of the amidine group with the SAM binding pocket, which is absent when the linker is not long enough. These results provide structural insights into developing the amidine type of PRMT1 inhibitors.


 Please wait while we load your content...
Please wait while we load your content...