Structure–activity relationships for the synthesis of selective cyclooxygenase 2 inhibitors: an overview (2009–2016)†
Abstract
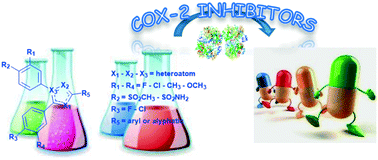
Most drugs used to treat pain and inflammation act through inhibition of the enzymes prostaglandin G/H synthase, commonly known as cyclooxygenase (COX). Among these, the simultaneous inhibition of cyclooxygenase 1 (COX-1) would explain the unwanted side effects in the gastrointestinal tract and many adverse cardiovascular effects, such as high blood pressure, myocardial infarction and thrombosis. These side effects led in time to the development of NSAIDs that behave as selective COX-2 inhibitors. This manuscript highlights the structure–activity relationships which characterize the chemical scaffolds endowed with selective COX-2 inhibition. Additionally, the role of COX-2 inhibitors in the pain phenomenon and cancer is discussed.


 Please wait while we load your content...
Please wait while we load your content...