Abstract
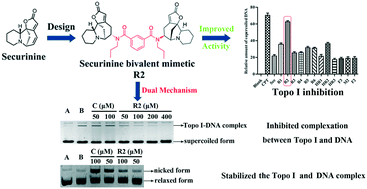
A series of novel bivalent securinine mimetics incorporating different linkers between C-15 and C-15′ were synthesized and their topoisomerase I (Topo I) inhibitory activities evaluated. It was thus revealed that mimetic R2 incorporating a rigid m-substituted benzene linker exhibits Topo I inhibitory activity three times that of parent securinine. Comprehensive structure–activity relationship analyses in combination with docking studies were used to rationalize the potent activity of these bivalent mimetics. Mechanistic studies served to confirm the deductions arising from docking studies that the active bivalent mimetics not only inhibited complexation between Topo I and DNA but also stabilized the Topo I–DNA complex itself.


 Please wait while we load your content...
Please wait while we load your content...