Abstract
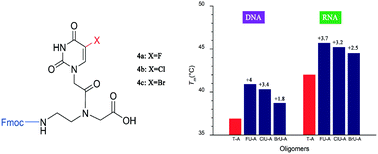
The monomers of peptide nucleic acids containing 5-halouracils (5-XU-PNA), incorporated into heptameric PNA in the middle position, have been synthesized. Thermodynamic analyses revealed that the heptameric PNA oligomer with DNA and RNA showed higher duplex stability compared to the unmodified PNA counterpart. NMR studies suggested that the electron withdrawing effect of the halogen atom increased the strength of the XU–A hydrogen bond.


 Please wait while we load your content...
Please wait while we load your content...