Synthesis, characterization, solubilization, cytotoxicity and antioxidant activity of aminomethylated dihydroquercetin†
Abstract
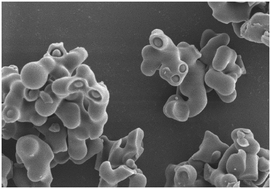
A dihydroquercetin derivative (DHQA) was prepared through aminomethylation to overcome the low water solubility and bioavailability of dihydroquercetin (DHQ). DHQA was characterized through HPLC, nuclear magnetic resonance, scanning electron microscopy, X-ray diffraction, and thermogravimetric analyses. DHQA was converted into the amorphous form, but the major structure of DHQ remained unchanged. Solubilization and dissolution tests were also performed. Results showed that the solubility and dissolution rates of DHQA were approximately 16.28 and 6.31 times higher than those of DHQ, respectively. The MTT assay of DHQA showed a non-toxic effect against non-cancerous HEK-293T cells (EC50 = 820.00 μM), and potent inhibitory activity against cancerous Hela cells (EC50 = 138.17 μM). Finally, the antioxidant activity of DHQA was confirmed in vitro through DPPH and ABTS radical scavenging activity assays. DHQA displayed high antioxidant activities with low IC50 values (0.043 and 0.042 mM, respectively). Reducing Fe3+ power assay indicated that DHQA exhibited higher reducing power than DHQ and ascorbic acid.

 Please wait while we load your content...
Please wait while we load your content...