The nucleosomal surface is the main target of histone ADP-ribosylation in response to DNA damage
Abstract
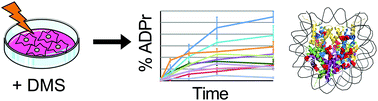
ADP-ribosylation is a protein post-translational modification catalyzed by ADP-ribose transferases (ARTs). ART activity is critical in mediating many cellular processes, and is required for DNA damage repair. All five histone proteins are extensively ADP-ribosylated by ARTs upon induction of DNA damage. However, how these modifications aid in repair processes is largely unknown, primarily due to lack of knowledge about where they site-specifically occur on histones. Here, we conduct a comprehensive analysis of histone Asp/Glu ADP-ribosylation sites upon DNA damage induced by dimethyl sulfate (DMS). We also demonstrate that incubation of cell nuclei with NAD+, as has been done previously in the literature, leads to spurious ADP-ribosylation levels of histone proteins. Altogether, we were able to identify 30 modification sites, 20 of which are novel. We also quantify the abundance of these modification sites during the course of DNA damage insult to identify which sites are critical for mediating repair. We found that every quantifiable site increases in abundance over time and that each identified ADP-ribosylation site is located on the surface of the nucleosome. Together, the data suggest specific Asp/Glu residues are unlikely to be critical for DNA damage repair and rather that this process is likely dependent on ADP-ribosylation of the nucleosomal surface in general.


 Please wait while we load your content...
Please wait while we load your content...