Mechanism of the formation of the RecA–ssDNA nucleoprotein filament structure: a coarse-grained approach
Abstract
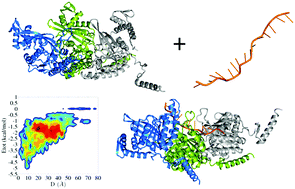
In prokaryotes, the RecA protein catalyzes the repair and strand exchange of double-stranded DNA. RecA binds to single-stranded DNA (ssDNA) and forms a presynaptic complex in which the protein polymerizes around the ssDNA to form a right-handed helical nucleoprotein filament structure. In the present work, the mechanism for the formation of the RecA–ssDNA filament structure is modeled using coarse-grained molecular dynamics simulations. Information from the X-ray structure was used to model the protein itself but not its interactions; the interactions between the protein and the ssDNA were modeled solely by electrostatic, aromatic, and repulsive energies. For the present study, the monomeric, dimeric, and trimeric units of RecA and 4, 8, and 11 NT-long ssDNA, respectively, were studied. Our results indicate that monomeric RecA is not sufficient for nucleoprotein filament formation; rather, dimeric RecA is the elementary binding unit, with higher multimeric units of RecA facilitating filament formation. Our results reveal that loop region flexibility at the primary binding site of RecA is essential for it to bind the incoming ssDNA, that the aromatic residues present in the loop region play an important role in ssDNA binding, and that ATP may play a role in guiding the ssDNA by changing the electrostatic potential of the RecA protein.


 Please wait while we load your content...
Please wait while we load your content...