An enhanced hTERT promoter-driven CRISPR/Cas9 system selectively inhibits the progression of bladder cancer cells†
Abstract
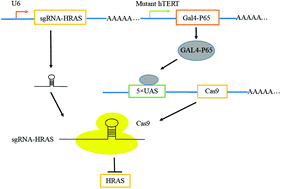
The current therapies for treating tumors are lacking in efficacy and specificity. Synthetic biology principles may bring some new possible methods for curing cancer. Here we present a synthetic logic circuit based on the CRISPR/Cas9 system. The CRISPR/Cas9 technology has been applied in many biological fields, including cancer research. In this study, the expression of Cas9 nuclease was controlled indirectly by an enhanced hTERT promoter using the GAL4/upstream activating sequence (UAS) binding system. Cas9 was driven by 5XUAS, single guide RNA (sgRNA) was used to target mutant or wild-type HRAS, and the fusion gene GAL4-P65 was driven by the enhanced hTERT promoter. The system was tested in bladder cancer cells (T24 and 5637) and the results showed that the enhanced hTERT promoter could drive the expression of GAL4-P65 in these bladder cancer cell lines. Then all these devices were packed into lentivirus and the results of quantitative real-time PCR showed that the mRNA expression level of HRAS was selectively inhibited in the T24 and 5637 cells. The results of functional experiments suggested that the proliferation, cell migration and invasion were selectively suppressed, and that the apoptosis rate was increased in bladder cancer cells but not in human foreskin fibroblasts (HFF). In conclusion, we successfully constructed an enhanced hTERT promoter-driven CRISPR/Cas9 system and data showed that it could selectively suppress the progression of bladder cancer cells.


 Please wait while we load your content...
Please wait while we load your content...