Global DNA dynamics of 8-oxoguanine repair by human OGG1 revealed by stopped-flow kinetics and molecular dynamics simulation†
Abstract
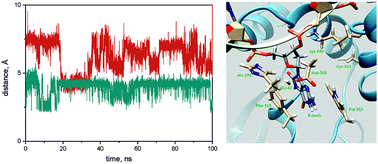
The toxic action of different endogenous and exogenous agents leads to damage in genomic DNA. 8-Oxoguanine is one of the most often generated and highly mutagenic oxidative forms of damage in DNA. Normally, in human cells it is promptly removed by 8-oxoguanine-DNA-glycosylase hOGG1, the key DNA-repair enzyme. An association between the accumulation of oxidized guanine and an increased risk of harmful processes in organisms was already found. However, the detailed mechanism of damaged base recognition and removal is still unclear. To clarify the role of active site amino acids in the damaged base coordination and to reveal the elementary steps in the overall enzymatic process we investigated hOGG1 mutant forms with substituted amino acid residues in the enzyme base-binding pocket. Replacing the functional groups of the enzyme active site allowed us to change the rates of the individual steps of the enzymatic reaction. To gain further insight into the mechanism of hOGG1 catalysis a detailed pre-steady state kinetic study of this enzymatic process was carried out using the stopped-flow approach. The changes in the DNA structure after mixing with enzymes were followed by recording the FRET signal using Cy3/Cy5 labels in DNA substrates in the time range from milliseconds to hundreds of seconds. DNA duplexes containing non-damaged DNA, 8-oxoG, or an AP-site or its unreactive synthetic analogue were used as DNA-substrates. The kinetic parameters of DNA binding and damage processing were obtained for the mutant forms and for WT hOGG1. The analyses of fluorescence traces provided information about the DNA dynamics during damage recognition and removal. The kinetic study for the mutant forms revealed that all introduced substitutions reduced the efficiency of the hOGG1 activity; however, they played pivotal roles at certain elementary stages identified during the study. Taken together, our results gave the opportunity to restore the role of substituted amino acids and main “damaged base–amino acid” contacts, which provide an important link in the understanding the mechanism of the DNA repair process catalyzed by hOGG1.


 Please wait while we load your content...
Please wait while we load your content...