Dynamic properties of dipeptidyl peptidase III from Bacteroides thetaiotaomicron and the structural basis for its substrate specificity – a computational study†
Abstract
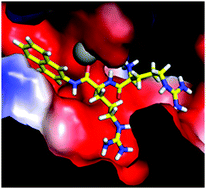
Dipeptidyl peptidase III (DPP III) from the human gut symbiont Bacteroides thetaiotaomicron (Bt) is the first identified prokaryotic DPP III orthologue. It has low sequence similarity to the thoroughly studied human DPP III, and differently from eukaryotic orthologues it has a cysteine (Cys450) residue in the zinc-binding motif HEXXGH (HECLGH). The recently determined crystal structure of BtDPP III showed that its 3D structure, similar to the structure of the human DPP III, consists of two domains with a wide cleft in between. Although such a striking similarity of the 3D structures of orthologues with low sequence similarity is not surprising, it is no guarantee for similarity of their dynamic properties and the catalytic performance. Here, we report the results of the molecular modelling study of BtDPP III, wild type and its C450S mutant, as well as their complexes with characteristic DPP III substrates Arg–Arg–2-naphthylamide (RRNA) and Lys–Ala–2-naphtylamide (KANA). During several hundred nanoseconds of all-atom MD simulations of the wild type protein, the long range conformational changes, which can be described as protein ‘closing’, have been traced. We have determined a similar conformational change for the human orthologue as well. However, the amplitude of the change is lower for BtDPP III than for the human DPP III. The MD simulations have been performed using ff03, ff12SB and ff14SB force fields wherein the results of the last two better fit to the experimental results. The hydrogen bond analysis indicates reasons for higher substrate specificity of BtDPP III towards RRNA than KANA as well as for the decrease of the RRNA hydrolysis rate induced by the Cys450 to Ser mutation. The obtained results are in line with the experimental data.


 Please wait while we load your content...
Please wait while we load your content...