Functional roles of intrinsic disorder in CRISPR-associated protein Cas9
Abstract
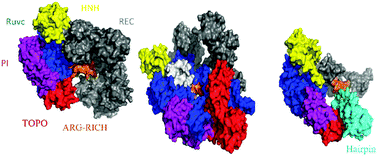
Protein intrinsic disorder is an important characteristic commonly detected in multifunctional or RNA- and DNA-binding proteins. Due to their high conformational flexibility and solvent accessibility, intrinsically disordered proteins (IDPs) and IDP regions (IDPRs) execute diverse functions including interaction with multiple partners, and are frequently subjected to various post-translational modifications. Recent studies on the components comprising the CRISPR (clustered regularly interspaced short palindromic repeats) system have elucidated the crystal structure of Cas9 proteins and the mechanism by which the Cas9–sgRNA complex recognizes and cleaves its target DNA. Yet the extent and functional implications of intrinsic disorder in the Cas9 protein have never been fully assessed. Here, we present a comprehensive computational analysis based on both sequence and structural data in an attempt to investigate the roles of IDPRs in the functioning of Cas9 proteins of different origin. We conclude that among the functional roles of IDPRs in Cas9 proteins are recognition of the target DNA and mediation of nucleic acid and protein binding.


 Please wait while we load your content...
Please wait while we load your content...