Expediting dynamics approach to understand the influence of 14-3-3ζ causing metastatic cancer through the interaction of YAP1 and β-TRCP
Abstract
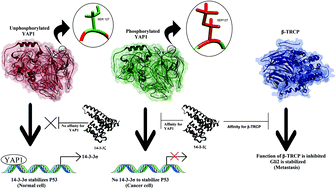
The 14-3-3ζ protein acts as a molecular switch in regulating the TGF-β pathway, which alters from a tumor suppressor in the early stage of breast cancer to a promoter of metastasis in the late stage. This change is due to the binding of 14-3-3ζ with YAP1 and β-TRCP in premalignant and cancer cells, respectively. Owing to this inappropriate role of 14-3-3ζ when involved in cancer and metastasis, we predicted that Gln15, Glu17, Tyr211, and Gln219 are hotspot residues of 14-3-3ζ during its interaction with YAP1 protein. Similarly, we identified Gln15, Tyr211, Leu216, and Leu220 as hotspot residues of 14-3-3ζ during its interaction with β-TRCP protein. Targeting these residues of 14-3-3ζ can prevent cancer and metastasis caused by malfunctioning of the TGF-β pathway. In this work, we also predicted that YAP1 is an intrinsically disordered protein (IDP), and such proteins bind with other proteins via either an induced fit or a conformational selection mechanism. Intuitively, we found that 14-3-3ζ has high affinity towards phosphorylated YAP1 at Ser127 rather than unphosphorylated YAP1, which is in close agreement with previously reported experimental works. Thus, we performed an analysis by molecular dynamics simulations to reveal the conformational changes in YAP1 after phosphorylation at the atomistic level. Our work clearly illustrates the effect of phosphorylation on YAP1 in terms of conformational changes and the regulation of its function.


 Please wait while we load your content...
Please wait while we load your content...