Mass spectrometry based identification of galectin-3 interacting proteins potentially involved in lung melanoma metastasis†‡
Abstract
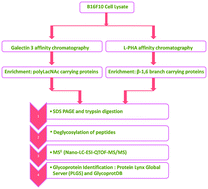
Adhesive interactions between molecules on tumor cells and those on target organs play a key role in organ specific metastasis. Poly-N-acetyl-lactosamine (polyLacNAc) substituted N-oligosaccharides on melanoma cell surface glycoproteins promote lung specific metastasis via galectin-3 by facilitating their arrest and extravasation. This study reports the identification and characterization of galectin-3 interacting proteins using a combination of galectin-3 sepharose affinity and leucoagglutinating phytohemagglutinin (L-PHA) columns. A total of 83 proteins were identified as galectin-3 interacting glycoproteins, of which 35 were constituents of the L-PHA bound fraction, suggesting that these proteins carry polyLacNAc substituted β1,6 branched N-glycans. The identities of some of these proteins, like LAMP-1, LAMP-3, basigin, embigin, and α5 and β1 Integrin, have been confirmed by western blotting, and functional relevance with respect to metastatic properties has been established.


 Please wait while we load your content...
Please wait while we load your content...