Protein stability and dynamics influenced by ligands in extremophilic complexes – a molecular dynamics investigation
Abstract
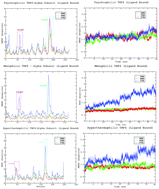
In this study, we explore the structural and dynamic adaptations of the Tryptophan synthase α-subunit in a ligand bound state in psychrophilic, mesophilic and hyperthermophilic organisms at different temperatures by MD simulations. We quantify the global and local fluctuations in the 40 ns time scale by analyzing the root mean square deviation/fluctuations. The distinct behavior of the active site and loop 6 is observed with the elevation of temperature. Protein stability relies more on electrostatic interactions, and these interactions might be responsible for the stability of varying temperature evolved proteins. The paper also focuses on the effect of temperature on protein dynamics and stability governed by the distinct behavior of the ligand associated with its retention, binding and dissociation over the course of time. The integration of principle component analysis and a free energy landscape was useful in identifying the conformational space accessible to ligand bound homologues and how the presence of the ligand alters the conformational and dynamic properties of the protein.


 Please wait while we load your content...
Please wait while we load your content...