Interaction and inhibitory influence of the azo dye carmoisine on lysozyme amyloid fibrillogenesis†
Abstract
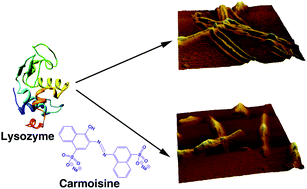
The binding of the common food colorant carmoisine and its inhibitory effect on amyloid fibrillation in lysozyme have been investigated. Since humans are increasingly exposed to various food colorants like carmoisine, such studies are highly relevant. In the presence of lysozyme, the carmoisine absorption spectrum exhibited hypochromic changes. The intrinsic fluorescence of lysozyme was also quenched on interaction. Time-resolved fluorescence results suggested that the binding mechanism involved ground state complexation. The binding was predominantly dominated by non-polyelectrolytic forces. The molecular distance between the donor (lysozyme) and the acceptor (carmoisine), calculated from FRET theory, was found to be 3.37 nm, indicating that carmoisine binds close to Trp-62/63 residues in the β-domain of the protein. Information on alterations in the microenvironment surrounding the Trp-residues was also obtained from synchronous fluorescence data. Carmoisine binding induced significant loss in the alpha helical organization of lysozyme. The binding, nevertheless, did not influence the thermal stability of lysozyme significantly. The binding reaction was exothermic and driven by large negative enthalpy and small but favourable entropic contributions. Thioflavin T assay, far-UV circular dichroism studies and AFM imaging profiles testified that carmoisine had a significant inhibitory effect on amyloid fibrillogenesis in lysozyme. Carmoisine also had a definitive defibrillating effect on existing fibrils. The results may provide new insights for designing new small molecule inhibitors for amyloid related diseases.


 Please wait while we load your content...
Please wait while we load your content...