Covalent inhibition of protein tyrosine phosphatases
Abstract
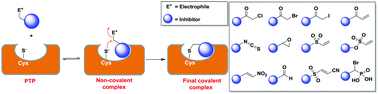
Protein tyrosine phosphatases (PTPs) are a large family of 107 signaling enzymes that catalyze the hydrolytic removal of phosphate groups from tyrosine residues in a target protein. The phosphorylation status of tyrosine residues on proteins serve as a ubiquitous mechanism for cellular signal transduction. Aberrant function of PTPs can lead to many human diseases, such as diabetes, obesity, cancer, and autoimmune diseases. As the number of disease relevant PTPs increases, there is urgency in developing highly potent inhibitors that are selective towards specific PTPs. Most current efforts have been devoted to the development of active site-directed and reversible inhibitors for PTPs. This review summarizes recent progress made in the field of covalent inhibitors to target PTPs. Here, we discuss the in vivo and in vitro inactivation of various PTPs by small molecule-containing electrophiles, such as Michael acceptors, α-halo ketones, epoxides, and isothiocyanates, etc. as well as oxidizing agents. We also suggest potential strategies to transform these electrophiles into isozyme selective covalent PTP inhibitors.


 Please wait while we load your content...
Please wait while we load your content...