Computational investigation of the human SOD1 mutant, Cys146Arg, that directs familial amyotrophic lateral sclerosis
Abstract
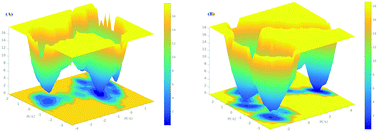
The genetic substitution mutation of Cys146Arg in the SOD1 protein is predominantly found in the Japanese population suffering from familial amyotrophic lateral sclerosis (FALS). A complete study of the biophysical aspects of this particular missense mutation through conformational analysis and producing free energy landscapes could provide an insight into the pathogenic mechanism of ALS disease. In this study, we utilized general molecular dynamics simulations along with computational predictions to assess the structural characterization of the protein as well as the conformational preferences of monomeric wild type and mutant SOD1. Our static analysis, accomplished through multiple programs, predicted the deleterious and destabilizing effect of mutant SOD1. Subsequently, comparative molecular dynamic studies performed on the wild type and mutant SOD1 indicated a loss in the protein conformational stability and flexibility. We observed the mutational consequences not only in local but also in long-range variations in the structural properties of the SOD1 protein. Long-range intramolecular protein interactions decrease upon mutation, resulting in less compact structures in the mutant protein rather than in the wild type, suggesting that the mutant structures are less stable than the wild type SOD1. We also presented the free energy landscape to study the collective motion of protein conformations through principal component analysis for the wild type and mutant SOD1. Overall, the study assisted in revealing the cause of the structural destabilization and protein misfolding via structural characterization, secondary structure composition and free energy landscapes. Hence, the computational framework in our study provides a valuable direction for the search for the cure against fatal FALS.


 Please wait while we load your content...
Please wait while we load your content...