Effects of flexibility and electrostatic interactions on the coupled binding–folding mechanisms of Chz.core and H2A.z–H2B†
Abstract
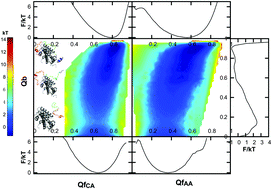
The intrinsically disordered protein (IDP) Chz.core, which is the interaction core of Chz1, shows binding preference to histone variant H2A.z. Although there are several studies on the binding process of Chz.core, the detailed coupled binding–folding processes are still elusive. In this study, we explored the coupled binding–folding mechanism and the effect of flexibility by continuously monitoring the flexibility degree of Chz.core. We applied an all-atom structure-based model (SBM), which takes advantage of providing both backbone and sidechain information about the conformational changes of Chz.core during binding. We presented a somewhat different “fly-casting” picture that the long IDP can undergo a tertiary stretching and bending with larger capture radii than ordered proteins. Our results suggest that the higher flexibility of Chz.core contributes to the shorter times for capturing events, leading to higher recognition efficiencies. In addition, compared to the ordered proteins, the high flexibility of the intrinsically disordered protein enables Chz.core to have a lower binding barrier and a faster association rate, which are favorable for the binding process to its partner H2A.z–H2B.


 Please wait while we load your content...
Please wait while we load your content...