Molecular dynamics simulations elucidate conformational selection and induced fit mechanisms in the binding of PD-1 and PD-L1†
Abstract
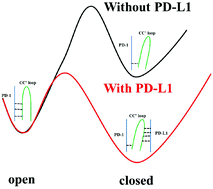
Blockage of the interactions between immunologic checkpoint protein PD-1 and its ligand PD-L1 showed efficacy for cancer treatment. X-ray structures have captured static conformational snapshots of PD-1 and revealed that the CC′ loop adopts an open conformation in the apo-protein but turns into a closed form and interacts with PD-L1 in the complex. This structural heterogeneity brings difficulties for structure-based drug discovery targeting PD-1. To gain insights into the role of the CC′ loop in molecular recognition, we have undertaken a comparative study between the open and closed conformations in apo-PD-1 and the PD-1/PD-L1 complex using molecular dynamics simulations. Results show that the moderate stability of intramolecular hydrogen bonds between SER71 and THR120 allows the CC′ loop to sample both the open and closed states in apo-PD-1. Binding of PD-L1 accelerates the open-to-closed switch and locks the loop in the closed state through four newly formed intermolecular hydrogen bonds. Thus, we suggest a complex binding mechanism between PD-1 and PD-L1 where both the conformational selection and induced fit theories play a role.


 Please wait while we load your content...
Please wait while we load your content...