The antiproliferative activity of di-2-pyridylketone dithiocarbamate is partly attributed to catalase inhibition: detailing the interaction by spectroscopic methods†
Abstract
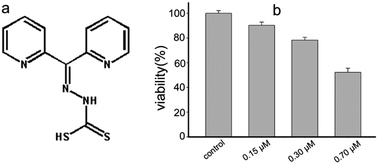
The bioactivity of drugs is attributed to their interaction with biological molecules, embodied in either their direct or indirect influence on enzyme activity and conformation. Di-2-pyridylketone hydrazine dithiocarbamate (DpdtC) exhibits significant antitumor activity in our preliminary study. We speculated that its activity may partly stem from enzyme inhibition due to strong metal chelating ability. To this end, we assessed its effect on catalase from erythrocytes and found evidence of inhibition, which was further confirmed by ROS determination in vivo. Thus, detailing the interaction between the agent and catalase via spectroscopic methods and molecular docking was required to obtain information on both the dynamics and thermodynamic parameters. The Lineweaver–Burk plot implied an uncompetitive pattern between DpdtC and catalase from beef liver, and IC50 = ∼7 μM. The thermodynamic parameters from fluorescence quenching measurements indicated that DpdtC could bind to catalase with moderate affinity (Ka = approximately 104 M−1). CD spectra revealed that DpdtC could significantly disrupt the secondary structure of catalase. Docking studies indicated that DpdtC bound to a flexible region of catalase, involving hydrogen bonds and salt bond; this was consistent with thermodynamic results from spectral investigations. Our data clearly showed that catalase inhibition of DpdtC was not due to direct chelation of iron from heme (killing), but through an allosteric effect. Thus, it can be concluded that the antiproliferative activity of DpdtC is partially attributed to its catalase inhibition.


 Please wait while we load your content...
Please wait while we load your content...