Screening methods for identifying pharmacological chaperones
Abstract
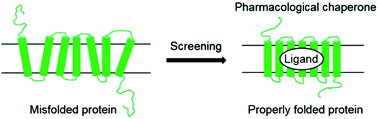
Protein folding is crucial for most proteins to achieve their correct three-dimensional conformations and function properly. Defects in protein folding frequently caused by mutations lead to a range of protein misfolding diseases, including Alzheimer’s disease, Parkinson’s disease, cystic fibrosis, amyloidosis, Gaucher disease, etc. One approach to treat these devastating diseases would be to use pharmacological chaperones, which are small-molecules that bind to and stabilize misfolded proteins, thereby correcting their pathogenic misfolding and rescuing their functions. As such, pharmacological chaperone therapy holds great promise for the treatment of numerous protein misfolding diseases. In this review, we highlight recent strategies for identifying small-molecules that act as pharmacological chaperones and revert protein misfolding diseases, with a focus on reports within the last five years.


 Please wait while we load your content...
Please wait while we load your content...