Molecular dynamics investigations of membrane-bound CYP2C19 polymorphisms reveal distinct mechanisms for peripheral variants by long-range effects on the enzymatic activity†
Abstract
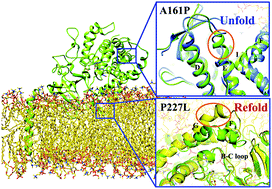
Increasing sophistication in methods used to account for human polymorphisms in susceptibility to drug metabolism has been one of the success stories in the prevention of adverse drug reactions. Genetic polymorphisms in drug-metabolizing enzymes can affect enzyme activity and cause differences in treatment response or drug toxicity. CYP2C19 is one of the most highly polymorphic CYP enzymes and acts on 10–15% of drugs in current clinical use. Despite the number of experimental analyses carried out for this system, the detailed structural basis for altered catalytic properties of polymorphic CYP2C19 variants remains unexplained at the atomic level. To this end, we have investigated the mutation effects on structural characteristics and tunnel geometry upon single point mutations to elucidate the underlying molecular mechanism for the enzymatic activity deficiencies by using the fully atomistic molecular dynamics (MD) simulations in their native, membrane-bound cellular environment. The obtained results demonstrate how significant sequence divergence causes heterogeneous variations, and further affects the shape and chemical properties of the substrate binding site. Principal component analysis (PCA) results combined with free energy calculations have revealed distinct mechanisms for different peripheral variants, implying a more complicated process for the decrease/loss of enzymatic activity upon the introduction of point mutations in CYP2C19 rather than simply structural changes of the region where the mutation is located. Overall, our present study provides important insights into the current pharmacogenetic knowledge of human drug-metabolizing CYP2C19 to understand the large inter-individual variability in drug clearance. The knowledge of heterogeneous variations in structural features could guide future experimental and computational work on efficient and safe drug treatment with better pharmacokinetic properties based on the common variant alleles of CYP genes, which varies among different ethnic populations.


 Please wait while we load your content...
Please wait while we load your content...