Characterizing the structure–function relationship reveals the mode of action of a novel antimicrobial peptide, P1, from jumper ant Myrmecia pilosula†
Abstract
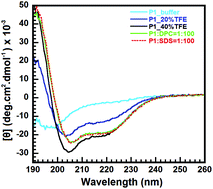
Microbial infections of antibiotic-resistant strains cause serious diseases and have a significant impact on public health worldwide, so novel antimicrobial drugs are urgently needed. Insect venoms, a rich source of bioactive components containing antimicrobial peptides (AMPs), are attractive candidates for new therapeutic agents against microbes. Recently, a novel peptide, P1, identified from the venom of the Australian jumper ant Myrmecia pilosula, showed potent antimicrobial activities against both Gram-negative and Gram-positive bacteria, but its structure–function relationship is unknown. Here, we used biochemical and biophysical techniques coupled with computational simulations to explore the mode of action of P1 interaction with dodecylphosphocholine (DPC) micelles as a model membrane system. Our circular dichroism (CD) and NMR studies revealed an amphipathic α-helical structure for P1 upon interaction with DPC micelles. A paramagnetic relaxation enhancement approach revealed that P1 orients its α-helix segment (F6–G14) into DPC micelles. In addition, the α-helix segment could be essential for membrane permeabilization and antimicrobial activity. Moreover, the arginine residues R8, R11, and R15 significantly contribute to helix formation and membrane-binding affinity. The lysine residue K19 of the C-terminus functionally guides P1 to interact with DPC micelles in the early interaction stage. Our study provides insights into the mode of action of P1, which is valuable in modifying and developing potent AMPs as antibiotic drugs.


 Please wait while we load your content...
Please wait while we load your content...