Effect of flexible linker length on the activity of fusion protein 4-coumaroyl-CoA ligase::stilbene synthase†
Abstract
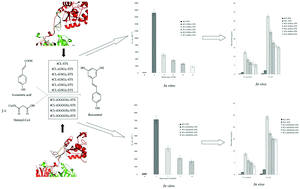
In order to elucidate the effect of flexible linker length on the catalytic efficiency of fusion proteins, two short flexible peptide linkers of various lengths were fused between Arabidopsis thaliana 4-coumaroyl-CoA ligase (4CL) and Polygonum cuspidatum stilbene synthase (STS) to generate fusion proteins 4CL–(GSG)n–STS (n ≤ 5) and 4CL–(GGGGS)n–STS (n ≤ 4). The fusion proteins were expressed in both Escherichia coli and Saccharomyces cerevisiae, and their bioactivities were tested in vitro and in vivo using purified proteins and engineered strains, respectively. The catalytic efficiency of the fusions decreased gradually with the increase of GSG or GGGGS repeats. In both engineered S. cerevisiae and E. coli in vivo experiments, the capacity of resveratrol production decreased gradually with increasing linker length. In silico analysis showed that the prediction of homology models of fusion proteins was consistent with the in vitro and in vivo results.


 Please wait while we load your content...
Please wait while we load your content...