Deformability-based microfluidic separation of pancreatic islets from exocrine acinar tissue for transplant applications†
Abstract
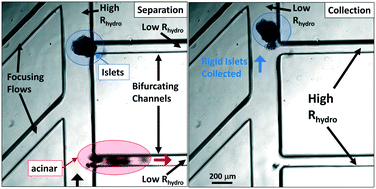
The long-term management of type-1 diabetes (T1D) is currently achieved through lifelong exogenous insulin injections. Although there is no cure for T1D, transplantation of pancreatic islets of Langerhans has the potential to restore normal endocrine function versus the morbidity of hypoglycemic unawareness that is commonly associated with sudden death among fragile diabetics. However, since endocrine islet tissues form a small proportion of the pancreas, sufficient islet numbers can be reached only by combining islets from multiple organ donors and the transplant plug contains significantly high levels of exocrine acinar tissue, thereby exacerbating immune responses. Hence, lifelong administration of immunosuppressants is required after transplantation, which can stress islet cells. The density gradient method that is currently used to separate islets from acinar tissue causes islets to be sparsely distributed over the centrifuged bins, so that the transplant sample obtained by combining multiple bins also contains significant acinar tissue levels. We show that in comparison to the significant size and density overlaps between the islet and acinar tissue populations post-organ digestion, their deformability overlaps are minimal. This feature is utilized to design a microfluidic separation strategy, wherein tangential flows enable selective deformation of acinar populations towards the bifurcating waste stream and sequential switching of hydrodynamic resistance enables the collection of rigid islets. Using 25 bifurcating daughter channels, a throughput of ∼300 islets per hour per device is obtained for enabling islet enrichment from relatively dilute starting levels to purity levels that meet the transplant criteria, as well as to further enhance islet purity from samples following density gradient enrichment. Based on confirmation of viability and functionality of the microfluidic-isolated islets using insulin secretion analysis and an angiogenesis assay, we envision utilizing this strategy to generate small-volume transplant plugs with high islet purity and significantly reduced acinar levels for minimizing immune responses after transplantation.
- This article is part of the themed collection: Lab on a Chip Recent Open Access Articles


 Please wait while we load your content...
Please wait while we load your content...