A tuneable microfluidic system for long duration chemotaxis experiments in a 3D collagen matrix†
Abstract
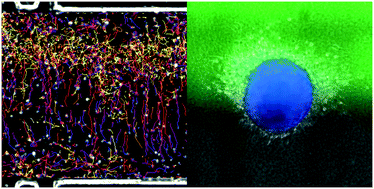
In many cell types, migration can be oriented towards a chemical stimulus. In mammals, for example, embryonic cells migrate to follow developmental cues, immune cells migrate toward sites of inflammation, and cancer cells migrate away from the primary tumour and toward blood vessels during metastasis. Understanding how cells migrate in 3D environments in response to chemical cues is thus crucial to understanding directed migration in normal and disease states. To date, chemotaxis in mammalian cells has been primarily studied using 2D migration models. However, it is becoming increasingly clear that the mechanisms by which cells migrate in 2D and 3D environments dramatically differ, and cells in their native environments are confronted with a complex chemical milieu. To address these issues, we developed a microfluidic device to monitor the behaviour of cells embedded in a 3D collagen matrix in the presence of complex concentration fields of chemoattractants. This tuneable microsystem enables the generation of (1) homogeneous, stationary gradients set by a purely diffusive mechanism, or (2) spatially evolving, stationary gradients, set by a convection–diffusion mechanism. The device allows for stable gradients over several days and is large enough to study the behaviour of large cell aggregates. We observe that primary mature dendritic cells respond uniformly to homogeneous diffusion gradients, while cell behaviour is highly position-dependent in spatially variable convection–diffusion gradients. In addition, we demonstrate a directed response of cancer cells migrating away from tumour-like aggregates in the presence of soluble chemokine gradients. Together, this microfluidic device is a powerful system to observe the response of different cells and aggregates to tuneable chemical gradients.


 Please wait while we load your content...
Please wait while we load your content...