WAT-on-a-chip: a physiologically relevant microfluidic system incorporating white adipose tissue
Abstract
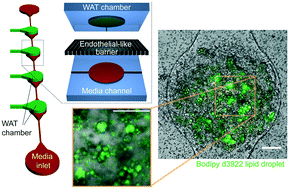
Organ-on-a-chip systems possess a promising future as drug screening assays and as testbeds for disease modeling in the context of both single-organ systems and multi-organ-chips. Although it comprises approximately one fourth of the body weight of a healthy human, an organ frequently overlooked in this context is white adipose tissue (WAT). WAT-on-a-chip systems are required to create safety profiles of a large number of drugs due to their interactions with adipose tissue and other organs via paracrine signals, fatty acid release, and drug levels through sequestration. We report a WAT-on-a-chip system with a footprint of less than 1 mm2 consisting of a separate media channel and WAT chamber connected via small micropores. Analogous to the in vivo blood circulation, convective transport is thereby confined to the vasculature-like structures and the tissues protected from shear stresses. Numerical and analytical modeling revealed that the flow rates in the WAT chambers are less than 1/100 of the input flow rate. Using optimized injection parameters, we were able to inject pre-adipocytes, which subsequently formed adipose tissue featuring fully functional lipid metabolism. The physiologically relevant microfluidic environment of the WAT-chip supported long term culture of the functional adipose tissue for more than two weeks. Due to its physiological, highly controlled, and computationally predictable character, the system has the potential to be a powerful tool for the study of adipose tissue associated diseases such as obesity and type 2 diabetes.


 Please wait while we load your content...
Please wait while we load your content...