Mimicking liver sinusoidal structures and functions using a 3D-configured microfluidic chip†
Abstract
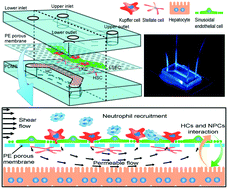
Physiologically, four major types of hepatic cells – the liver sinusoidal endothelial cells, Kupffer cells, hepatic stellate cells, and hepatocytes – reside inside liver sinusoids and interact with flowing peripheral cells under blood flow. It is hard to mimic an in vivo liver sinusoid due to its complex multiple cell–cell interactions, spatiotemporal construction, and mechanical microenvironment. Here we developed an in vitro liver sinusoid chip by integrating the four types of primary murine hepatic cells into two adjacent fluid channels separated by a porous permeable membrane, replicating liver's key structures and configurations. Each type of cells was identified with its respective markers, and the assembled chip presented the liver-specific unique morphology of fenestration. The flow field in the liver chip was quantitatively analyzed by computational fluid dynamics simulations and particle tracking visualization tests. Intriguingly, co-culture and shear flow enhance albumin secretion independently or cooperatively, while shear flow alone enhances HGF production and CYP450 metabolism. Under lipopolysaccharide (LPS) stimulations, the hepatic cell co-culture facilitated neutrophil recruitment in the liver chip. Thus, this 3D-configured in vitro liver chip integrates the two key factors of shear flow and the four types of primary hepatic cells to replicate key structures, hepatic functions, and primary immune responses and provides a new in vitro model to investigate the short-duration hepatic cellular interactions under a microenvironment mimicking the physiology of a liver.


 Please wait while we load your content...
Please wait while we load your content...