Incorporation of 67Zn and 68Zn into carbonic anhydrase: effects on isotope enrichment and enzymatic aspects
Abstract
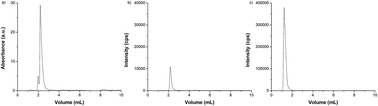
An enrichment of carbonic anhydrase (CA) with zinc isotopes (67Zn and 68Zn) is carried out to evaluate protein binding with a specific isotope. For this task, apo-CA is prepared with ortho-phenanthroline (OP) thus formatting the Zn–OP complex, and allowing the study of metal binding at CA. After an ultrafiltration procedure, the Zn–OP complex is removed, and the apo-CA is obtained, which is used for incubation with different zinc isotopes. An inductively coupled plasma mass spectrometer equipped with a dynamic reaction cell (DRC) is used for speciation purposes focusing on isotope ratio measurements. The optimization of instrumental parameters results in trueness measurements closer to natural conditions for Zn isotope ratios. The obtained precision is within the range observed for the same kind of m/z analyzer. To address the purity of apo-carbonic anhydrase, the protein was chromatographically separated using size exclusion with UV/Vis and ICP-MS detection. The incubation of CA with Zn isotopes changes the natural abundance of zinc isotopes, increasing the isotope ratios by 3.0 and 1.4 times for 67Zn and 68Zn, respectively, when compared to IRMM-3702. Such Zn-isotopically enriched CA was characterized, containing 1 atom of Zn per mole of CA. In terms of enzymatic activity, CA is enriched with different Zn isotopes yielding similar statistical results, producing 283 ± 32 nmol, 283 ± 11 nmol and 259 ± 32 nmol of p-nitrophenol for CA incubated with IRMM-3702, 67Zn and 68Zn, respectively. As significant differences were observed among apo and holo forms of CA, the enriched protein showed great potential for use in speciation analysis studies.
- This article is part of the themed collection: In memory of Joe Caruso


 Please wait while we load your content...
Please wait while we load your content...