Direct analysis of gaseous mercury in ambient air by gas to particle conversion-gas exchange ICPMS
Abstract
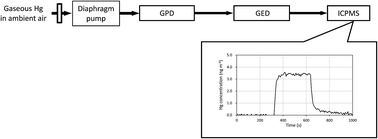
For the first time, direct analysis of gaseous mercury (Hg) at a concentration level of a few ng m−3 in ambient air using the gas to particle conversion-gas exchange technique, coupled with inductively coupled plasma mass spectrometry (ICPMS) has been achieved. Gaseous Hg was converted to mercury oxide (HgO) and agglomerated with ammonium nitrate (NH4NO3) particles by a gas to particle conversion device (GPD). The agglomerates were consequently introduced and measured by ICPMS through a gas exchange device (GED). The time-resolved signals from gaseous Hg in ambient air could be obtained by GPD–GED–ICPMS with a 10 ms dwell time for ICPMS, which demonstrated the real-time monitoring of gaseous Hg with high temporal resolution. No sample pretreatment procedures such as pre-concentration or separation of gaseous Hg from air, were needed before the introduction of gaseous Hg into GPD–GED–ICPMS. Longer dwell times of the ICPMS, such as 100 ms, 500 ms and 1000 ms revealed more stable time-resolved signals. A limit of detection (LOD) for gaseous Hg from the calibration curve was estimated to be ca. 0.12 ng m−3 when a longer dwell time of 1000 ms was selected. Since the LOD observed was sufficiently low compared to the background concentration level of ca. 2 ng m−3 in Japan, the GPD–GED–ICPMS technique is expected to be used for highly sensitive, direct and real-time monitoring of gaseous Hg in ambient air as well as for working and living environments.


 Please wait while we load your content...
Please wait while we load your content...