A dual-docking microfluidic cell migration assay (D2-Chip) for testing neutrophil chemotaxis and the memory effect†
Abstract
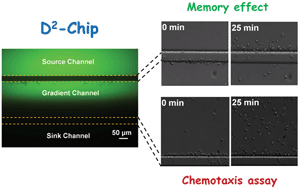
Chemotaxis is a classic mechanism for guiding cell migration and an important topic in both fundamental cell biology and health sciences. Neutrophils are a widely used model to study eukaryotic cell migration and neutrophil chemotaxis itself can lead to protective or harmful immune actions to the body. While much has been learnt from past research about how neutrophils effectively navigate through a chemoattractant gradient, many interesting questions remain unclear. For example, while it is tempting to model neutrophil chemotaxis using the well-established biased random walk theory, the experimental proof was challenged by the cell's highly persistent migrating nature. A special experimental design is required to test the key predictions from the random walk model. Another question that has interested the cell migration community for decades concerns the existence of chemotactic memory and its underlying mechanism. Although chemotactic memory has been suggested in various studies, a clear quantitative experimental demonstration will improve our understanding of the migratory memory effect. Motivated by these questions, we developed a microfluidic cell migration assay (so-called dual-docking chip or D2-Chip) that can test both the biased random walk model and the memory effect for neutrophil chemotaxis on a single chip enabled by multi-region gradient generation and dual-region cell alignment. Our results provide experimental support for the biased random walk model and chemotactic memory for neutrophil chemotaxis. Quantitative data analyses provide new insights into neutrophil chemotaxis and memory by making connections to entropic disorder, cell morphology and oscillating migratory response.


 Please wait while we load your content...
Please wait while we load your content...