Quantifying 3D chemotaxis in microfluidic-based chips with step gradients of collagen hydrogel concentrations†
Abstract
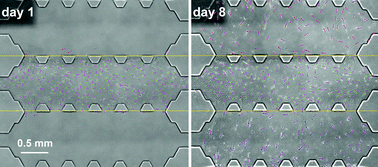
Cell migration is an essential process involved in crucial stages of tissue formation, regeneration or immune function as well as in pathological processes including tumor development or metastasis. During the last few years, the effect of gradients of soluble molecules on cell migration has been widely studied, and complex systems have been used to analyze cell behavior under simultaneous mechano-chemical stimuli. Most of these chemotactic assays have, however, focused on specific substrates in 2D. The aim of the present work is to develop a novel microfluidic-based chip that allows the long-term chemoattractant effect of growth factors (GFs) on 3D cell migration to be studied, while also providing the possibility to analyze the influence of the interface generated between different adjacent hydrogels. Namely, 1.5, 2, 2.5 and 4 mg ml−1 concentrations of collagen type I were alternatively combined with 5, 10 or 50 ng ml−1 concentrations of PDGF and VEGF (as a negative control). To achieve this goal, we have designed a new microfluidic device including three adjacent chambers to introduce hydrogels that allow the generation of a collagen concentration step gradient. This versatile and simple platform was tested by using dermal human fibroblasts embedded in 3D collagen matrices. Images taken over a week were processed to quantify the number of cells in each zone. We found, in terms of cell distribution, that the presence of PDGF, especially in small concentrations, was a strong chemoattractant for dermal human fibroblasts across the gels regardless of their collagen concentration and step gradient direction, whereas the effects of VEGF or collagen step gradient concentrations alone were negligible.


 Please wait while we load your content...
Please wait while we load your content...