A strategy of ketalization for the catalytic selective dehydration of biomass-based polyols over H-beta zeolite†
Abstract
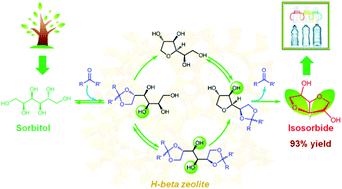
Biomass contains plentiful hydroxyl groups that lead to an oxygen-rich structure compared to petroleum-based chemicals. Dehydration is the most energy-efficient technique to remove oxygen; however, multiple similar vicinal hydroxyl groups in sugar alcohols impose significant challenges for their selective dehydration. Here, we present a novel strategy to control the etherification site in sugar alcohols by the ketalization of the vicinal-diol group for the highly selective formation of tetrahydrofuran derivatives. A ketone firstly reacts with terminal vicinal hydroxyl groups to form the 1,3-dioxolane structure. This structure of the constrained 1,3-dioxolane ring would improve the accessibility of reactive groups to facilitate intramolecular etherification. As a better leaving group than water, the ketone can also promote intramolecular etherification. Consequently, a range of tetrahydrofuran derivatives are produced in excellent yields with the H-beta zeolite catalyst under mild reaction conditions. This strategy opens up new opportunities for the efficient upgrading of biomass via the modification or protection of hydroxyl groups.
- This article is part of the themed collection: 2018 Green Chemistry Hot Articles


 Please wait while we load your content...
Please wait while we load your content...