Transition-metal-free and organic solvent-free conversion of N-substituted 2-aminobiaryls into corresponding carbazoles via intramolecular oxidative radical cyclization induced by peroxodisulfate†
Abstract
An atom-economical and environmentally benign approach for the synthesis of N-substituted carbazoles from analogous 2-aminobiaryls using peroxodisulfate in water is reported. The reactions proceeded through an intramolecular oxidative radical cyclization of N-substituted 2-aminobiaryls with in situ reoxidation of the resulting radical species. When compared to known methods for the synthesis of N-substituted carbazoles from 2-amidobiaryls, this protocol is practical, efficient and does not require a transition metal catalyst or toxic organic solvents.
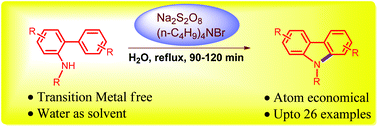


 Please wait while we load your content...
Please wait while we load your content...