Water promoted allylic nucleophilic substitution reactions of (E)-1,3 diphenylallyl acetate†
Abstract
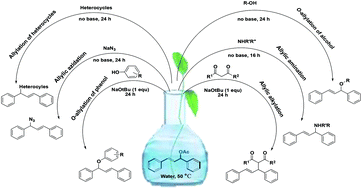
A transition metal free, water based, greener protocol for the allylic alkylation, allylic amination, O-allylation of (E)-1,3-diphenylallyl acetate is described. The developed methodology is applicable for a wide range of nucleophiles furnishing excellent yields of corresponding products up to 87% under mild reaction conditions. A distinct effect of water and base is explored for allylic nucleophilic substitution reactions of (E)-1,3-diphenylallyl acetate.


 Please wait while we load your content...
Please wait while we load your content...