Base-mediated tandem sulfonylation and oximation of alkenes in water†
Abstract
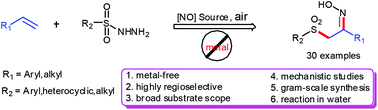
A base-mediated bifunctionalization of alkenes for the synthesis of α-sulfonylethanone oximes was developed in water under metal-free conditions. This reaction features a wide substrate scope and facile starting materials to afford the desired products in high yields.


 Please wait while we load your content...
Please wait while we load your content...