Iodine catalyzed oxidation of alcohols and aldehydes to carboxylic acids in water: a metal-free route to the synthesis of furandicarboxylic acid and terephthalic acid†
Abstract
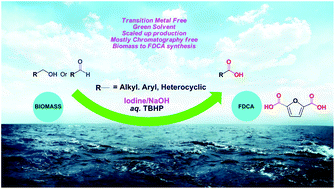
A metal-free iodine/NaOH-catalyzed oxidation of alcohols and aldehydes has been developed for the practical synthesis of a wide range of carboxylic acids using water as the solvent. This transformation involves dehydrogenation of an alcohol, followed by a fast attack of water on an aldehyde. This method is mostly free from chromatographic purification, which makes it suitable for large-scale synthesis. The iodine species formed during the reaction as the active oxidation catalyst has been deduced as IO2− by control experiments. We also demonstrate a 10 gram scale synthesis of furandicarboxylic acid (FDCA) from HMF in good yield using our method.


 Please wait while we load your content...
Please wait while we load your content...