Synthesis of urea derivatives via reductive carbon dioxide fixation into contracted porphyrin analogues†
Abstract
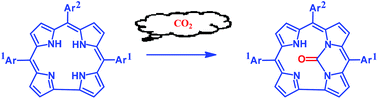
Carbon dioxide from ammonium carbonate is trapped by the free base corroles with the help of two adjacent inner NH groups (N21 and N22) and forms the N21,N22-carbamide-corrole derivatives. There is an extensive literature on functionalized ureas and their potent pharmaceutical applications. While the aliphatic, aromatic, alicyclic, and heterocyclic analogues of differently substituted ureas are well established, the N21,N22-carbamide-porphyrinoid derivatives have been reported rarely. Herein we present a new synthetic protocol to synthesize N21,N22-carbamide-corroles. These results thus open a greener avenue (without using any toxic chemicals like phosgene, CO, or isocyanate) for functionalized urea derivatives bearing porphyrinoid analogues.


 Please wait while we load your content...
Please wait while we load your content...