Highly efficient synthesis of β-nitrate ester carboxamides through the ring-opening of 2-oxazolines†
Abstract
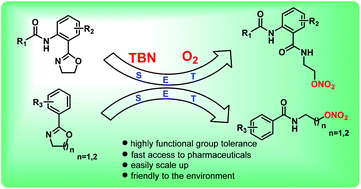
A novel method for the synthesis of β-nitrate ester carboxamides using non-corrosive tert-butyl nitrite (TBN) as the nitro source and easily available oxygen as the oxidant has been developed. Variously substituted 2-oxazolines were efficiently ring-opened to deliver the corresponding products in excellent yields. Notably, this reaction provides fast access to pharmaceuticals such as nicorandil.


 Please wait while we load your content...
Please wait while we load your content...