H-Bonding-promoted radical addition of simple alcohols to unactivated alkenes†
Abstract
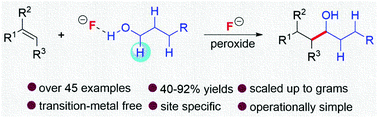
H-Bonding-induced radical addition of simple alcohols to unactivated olefins was achieved. It effectively solved the long-standing problems of reactivity and selectivity in this type of reaction. The hydroxyalkylation occurred via site-specific cleavage of the α-hydroxyl-C–H bond in alcohols. This method allows a highly atom-economical, operationally simple and environmentally benign access to diverse primary, secondary and tertiary alcohols, diols, and even polyfluorinated alcohols. These useful chemicals are traditionally synthesized by using commercially unavailable organometallics via complex operations. In contrast, they can be facilely obtained through this protocol utilizing widely available starting materials.


 Please wait while we load your content...
Please wait while we load your content...