Recycling of homogeneous catalysts in reactive ionic liquid – solvent-free aminofunctionalizations of alkenes†
Abstract
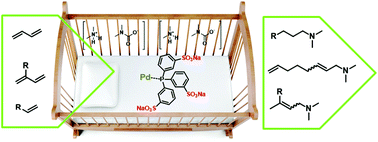
The catalyst in homogeneously catalyzed aminofunctionalizations is often difficult to recycle, making these reactions expensive on an industrial scale. The use of dimethylammonium dimethylcarbamate (dimcarb) as a reactive ionic liquid provides an elegant solution to this challenge, as it is a substrate and polar phase at the same time. In this work, homogeneously transition-metal catalyzed reactions – specifically hydroamination, telomerization and hydroaminomethylation – are carried out in neat substrates without additional solvents. The ionic character of dimcarb enables the immobilization of the active catalysts in the reactive ionic liquid, using sulfonated ligands. Investigations regarding the hydroamination of 1,3-dienes led to a total turnover number (TTON) of more than 8700 with β-farnesene in 12 repetitive recycling experiments. The telomerization of 1,3-butadiene was carried out over 30 consecutive runs without any loss of activity, resulting in a TTON of more than 90 000.


 Please wait while we load your content...
Please wait while we load your content...